
|
Environmental standard proposal
|
PollutionIntroductionAcute effects of pollution even in urban museums are confined to boxes and showcases. The usual culprit is acetic acid diffusing from wood but sometimes the artifacts cause their own, and neighbours' destruction. Cellulose nitrate and cellulose and polyvinyl acetates are common sources of pollutant gases. In the typical exhibition room with good ventilation, outdoor sources prevail. All impurities in air should be removed. There is no known safe concentration Humans can repair themselves, to some extent. Dead things cannot. We have tried to adapt pollution limits intended to preserve humans in a way entirely irrelevant to dead matter. However, this rule may not be correct. A saturated atmosphere of dibutylphthalate may stop stiffening and crazing of (once) flexible pvc. There have also been experiments with the use of gaseous corrosion inhibitors in drawers full of old iron. The free energy of reaction between gas and solid may change sign, and therefore reaction direction, at a low pollutant concentration. Such reversible reaction is specific to each reaction and cannot be used to refine a general standard. Ventilation rateThe optimum ventilation rate for reducing pollution damage is two air changes per hour Note that this ventilation can be provided by recirculation through a broad spectrum absorbent filter such as active carbon. This will reduce the cost of importing outside air and cleaning and conditioning it. Note also that this ventilation rate is sufficient to stop damage by re-absorption of pollutants generated by artifacts within the exhibition, or storage space. Note: ventilation regulations for public spaces may override this instruction Enclosures which have a deliberately slow ventilation rate, usually imposed to maintain RH stability and exclude dust, must exclude materials which generate volatile pollutants, even if the material is part of an artifact. Notes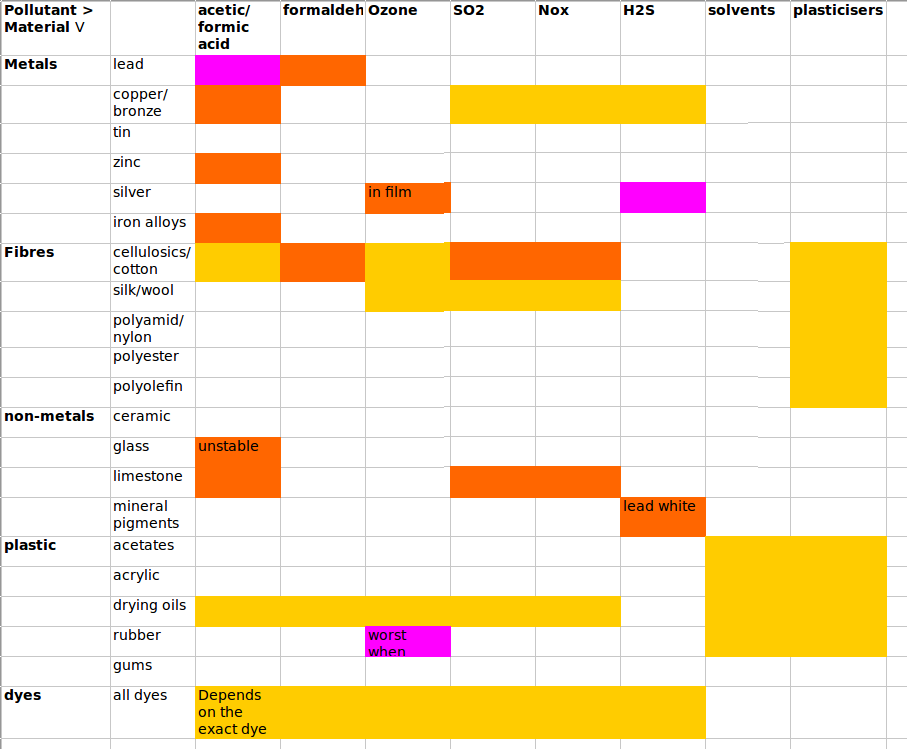 Anything with a yellow, or worse, in this diagram indicates a potential for rapid damage, so care must be taken to enclose the object in an inert container, with inert companion objects. Outdoor pollution concentrations can generally be obtained from government measuring programs. Limits quoted in standards are generally based on what can be measured rather than on proof of damage. Beware of research of doubtful technical quality. I refer particularly to acetic acid on lead, where, at low concentration, other passivating reactions have time to protect the lead, thus altering the substrate and giving the illusion of the reaction stopping on account of dilution alone. In olden times, church roofers travelled the country (in Denmark at least) melting the old lead and casting new sheets. These were then left to weather outside for six months before putting on the roof, thus reducing their susceptibility to corrosion by the oak spars of the roof construction. Pollution damage is nearly always observed in stagnant enclosures such as boxes, barrels, drawers and film cans. There is also in these enclosures evidence for activity of other pollutants, notably plasticisers which sometimes reach their saturation vapour pressure and condense as smears on the glass of picture frames, where it is best visible, but also of course on other surfaces where it is less visible. We also see crystals of antique pesticides decorating the glass of showcases. My non-quantitative suggestion is that internally generated pollution is vastly more damaging than stuff that drifts in from outside, at least in modern cities with some pretence of pollution control. The danger from enclosure results from the escape kinetics, which applies to all species of reasonably high vapour pressure. 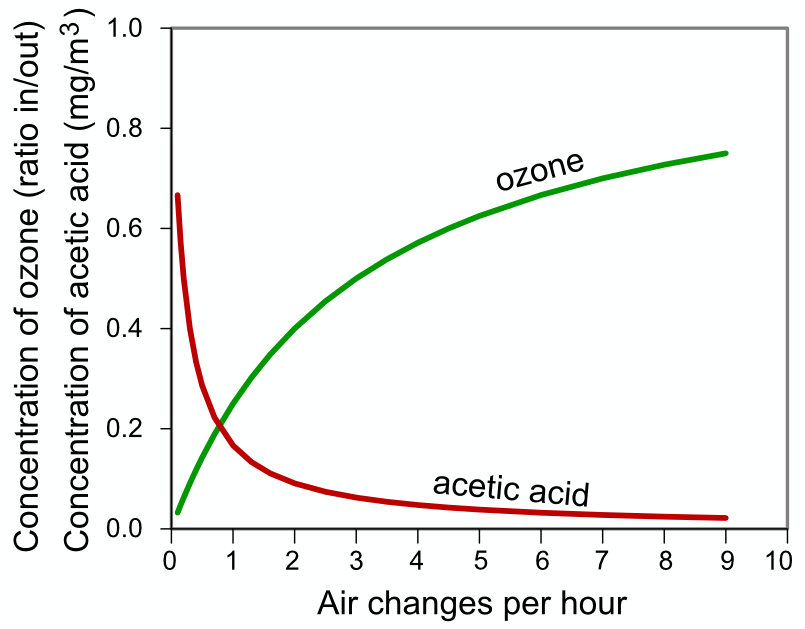 Notice the very steep rise in acetic acid concentration as air exchange drops below once per hour. A well sealed showcase is typically once per day. For an exhibition space, an air filtration rate better than once in two hours is not necessary. I haven't studied the kinetics of low volatile plasticisers. This would be an interesting exercise. List of destructive artifacts which need isolation or ventilationCellulose nitrate - widely used for film base, jewellery and domestic items like combs, 1920 - 1980(?) Cellulose acetate - almost universally used film base 1940 - 2000 Plywood urea formaldehyde glue - 1930 - 1990 (all dates are guesses - I am not a materials historian) wood - gives off acetic acid for ever soft plastics - exude low volatile plasticisers which condense on other surfaces. List of construction and decorative materials which emit volatilesWood in any form unless sealed with sheet metal Any polymer which contains plasticiser, or unreacted low molecular weight fraction (smell it). List of artifacts susceptible to airborne pollutantsAnything calcareous: limestone, marble, lime plaster - susceptible to acid gases, particularly if the artifact has an aqueous surface film due to hygroscopic salts or sugars. Lead - reacts with carboxylic acid gases silver - susceptible to H2S and other sulphur containing gases This pollution section needs improvement! |